Abstract
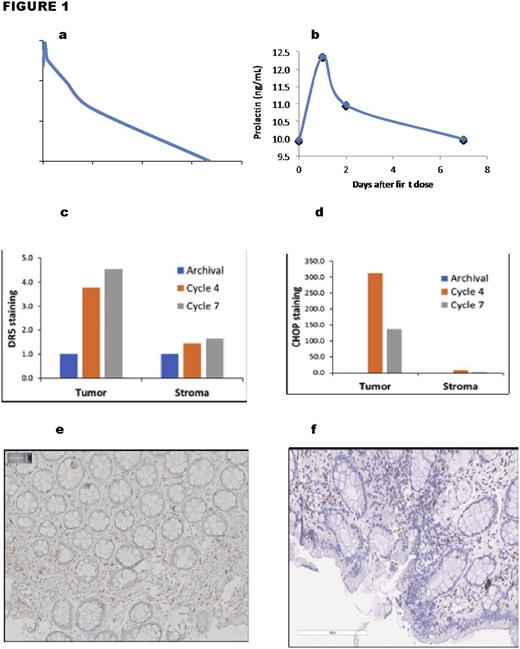
A heavily pretreated patient with mantle cell lymphoma in the gastrointestinal tract who was refractory to ibrutinib was treated with ONC201, a first in class selective dopamine receptor D2 (DRD2) antagonist, as part of a Phase I WIRB-approved dose-escalation trial (NCT02420795) for relapsed/refractory non-Hodgkin's lymphoma. Pre-treatment biopsies contained DRD2+DRD5- tumoral cells, a marker associated with sensitivity to ONC201. Beginning in April 2016, the patient received 125mg of ONC201 orally once every three weeks (one cycle). Pharmacokinetic analysis after start of treatment revealed a Cmax of ONC201 of 800ng/mL (~2 μM), with a half-life of 11.5 hours, and an area under the curve (AUC) of 9.2 h.ug/L (Figure 1A). After three 21-day cycles, all biopsied gastrointestinal areas were negative for lymphoma except for residual foci in the duodenum and rectum. During treatment, serum prolactin levels (Figure 1B) as well as both DR5 and CHOP in rectal tumoral cells (Figures 1C and D, respectively) were induced. These observations indicate that this patient received therapeutic exposure to ONC201 that resulted in target engagement in the tumor. In September 2016, after 7 cycles of ONC 201, a new lesion was detected in the ileum and sigmoid and the rectal biopsy showed persistent disease, prompting removal of the patient from the study and observation without further treatment. In March 2017, the patient was screened with esophagogastroduodenoscopy and colonoscopy and of 10 biopsied sited only one small remaining infiltrate was found in the rectal area. Concurrent PET/CT imaging, bone marrow biopsy with aspirate, and peripheral blood analyses failed to detect any disease by morphology, immunohistochemistry, and flow cytometry. Furthermore, sustained intratumoral induction of CHOP and DR5 were observed, and infiltration of CD45+ cells and CD8+ T cells were observed (figures E and F, respectively), fully 11 months after the first ONC201 dose. In conclusion, ONC201 is capable of inducing sustained pharmacodynamic effects that outlive its systemic presence and involves immune-stimulatory effects that may result in delayed, yet sustained, clinical activity in ibrutinib-refractory mantle cell lymphoma.
Lee: KITE PHARMA: Consultancy. Tarapore: Oncoceutics: Employment. Prabhu: Oncoceutics: Employment, Equity Ownership. Allen: Oncoceutics: Employment, Equity Ownership, Patents & Royalties. Schalop: Oncoceutics: Employment. Wang: Kite Pharma: Research Funding; Karus: Research Funding; Celgene: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Juno Therapeutic: Research Funding; Asana: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; BeiGene: Research Funding; Oncoceutics: Research Funding; Acerta Pharma: Consultancy, Honoraria, Research Funding; Oncternal: Research Funding; Novartis: Research Funding; Karyopharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal